Mass Percent of Water in Copper Sulfate
Simply so what is the mass percentage of water in copper sulfate. 1 Cu6355 gmol 1 S3207 gmol 4 O1600 gmol 15962 gmol Formula mass 15962 gmol 5 H20 1802 g H20mol 24972 gmol 2.

Percentage Composition Of H2o In Cuso4 5h2o Youtube
Molar masses were calculated using Molar Mass Calculator and rounded to four significant figures.

. By mass CuSO4 100 mCuSO4msample. Fourth calculate the percent of the mass in the hydrated compound that is from the 5 moles of H2O. WKT molar mass of Cuso4.
If you are given the formula you would do analysis of the information provided. DrBob222 Nov 24 2012 Respond to this Question Similar Questions. If you calculate the mass percent of water in your copper sulfate pentahydrate compound using your experimental data and find thaf it is too high what do you rhink happenex.
The hydrate is CopperII Sulfate or CuSO4 After conducting the experiment I found the mass percentage of this compound is. We review their content and use your feedback to keep the quality high. Experts are tested by Chegg as specialists in their subject area.
Who are the experts. Once you know the mass of copper sulphate you can calculate the mass percentage as follows. 4 rows Calculate the molar mass of CopperII Sulfate in grams per mole or search for a chemical.
F The division shows that there are 5 molecules of H2O on the original sample of CuSO 4. CuSO4xH2O where x is the number of hydrates Assume 100 g sample 284 g of water 716 g of copperII sulfate. Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass.
CuSO4 5 H 2O 24972g. What is the percentage of water of crystallization in CuSO4 5h2o. Show your work below.
C 3707 d 15962gmol e 001616moles of CuSO 4 008435moles of H 2 O. Commercial copper sulfate is usually about 98 pure copper sulfate and may contain traces of water. 16 g 34 Conclusion The percentage of water in Copper Sulfate was found to be 34.
Experimental value - actual value error actual value X 100. Why are hydrates important. 5H2O 159609 90 250.
Mass of water 095 g Mass of anhydrous copper sulfate 219 g Mass of hydrate copper sulfate 314 g ℹ A. Copper Sulfates Water of Hydration Lab. B Mass of dried CuSO 4 sample 258g.
Purpose To observe the effect of removing the water of hydration from hydrated copperII sulfate. Molar mass of copper Molar mass of copper sulfate 100 Explanation. What is the percent water in copperII sulfate pentahydrate CuSO4 5 H2O.
To calculate the percent of water you will divide the change in mass of your sample mass of water removed by the mass of the hydrated salt original mass. This results in the hydrate formula of CuSO4 5H20. In the formula CuSO 4 x H 2 O x is calculated as the amount of water of hydration divided by the amount of anhydrous copper sulfate.
It also means that copper sulfate pentahydrate contains 100 - 6392 3608 percent water by mass. Large crystals 1040 mm small crystals 210 mm snow crystals less than 2 mm and. Answered Calculate the percent by mass of water in copper II sulfate pentahydrate.
The class data for this lab show a similar result with the average water lost being 0365g and the percentage by. Anhydrous copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Mass of water 1802 x 5 901g.
Calculate the error for each trial. Note that the anhydrous salt is white but it dissolves in water to give a beautiful blue solution. Best Answer Copy there are 1596g of cupric sulfate and 901g of waters635gCu321gS 1604gO1596gCuSO4 5 1012gH5 160gO901g5H20 therefore the gram formula mass is 2497g the.
Chemistry Determine the percent by mass of water in your sample of hydrated copper II sulfate. Mass of H 2 O 152g. Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass.
The percent by mass of water of hydration is calculated as the mass of water of hydration divided by the mass of hydrated copper sulfate. H2O mass HO x 100 mass of hydrate The actual percentage of water in copperII sulfate pentahydrate is 360. CuSO4 5H20 Round to three sig figs 1 See answer Advertisement laaco is.
6355 g mol1 6355 3206 4 15999 g mol1 100. When determining the formula mass for a hydrate the waters of hydration must be included. Percentage of Water Amount of water in Copper Sulfate Pentahydrate Mass of Copper Sulfate Pentahydrate used Percentage of water.
Four types of crystal size are provided based on its usage. Mass 5 H2O mass 1 CuSO4 5 H2O x 100. Nov 24 2012 H2O mass H2Omass sample100.
In extension the percentage of water in the hydrated copper II sulfate compound was 3215. Up to 24 cash back The calculated mass of water lost from the hydrated copper II sulfate compound was 0451. Mass of water of crystallization 5 18 90.
284 water 716 copperII sulfate This is how I find the number of hydrates in the solution. And the hydrated copper. A Original mass has mass of water included while the final product has no mass of water since it has been lost by heating.

Ob Cuso4 5h2o Lab And Some Practice At Comp By Mass

Best Ammonium Copper Ii Sulfate Hexahydrate Nh4 2cu So4 2 6h2o Crystalline Copper Pure Products Chemical Formula

Calculating The Percent Water In A Hydrate Mr Pauller Youtube

Thermochemistry Presentation Specific Heat Enthalpy Heat Curve Practices Worksheets Presentation Molecular

Complete Density Of Penny Lab Presentation Spreadsheet Quiz Teaching Middle School Science Middle School Science Chemistry Labs

Friday March 6 Objective Determine The Ratio Of Water Molecules To Copper Sulfate Molecules In A Blue Chemical Checkpoint How Many Moles In 3 54 Grams Ppt Download

Redox Reactions Lab Activity Redox Reactions Lab Activities Chemical Equation

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Molar Mass Molecular Weight Of Cuso4 5h2o Copper Ii Sulfate Pentahydrate Youtube

Chemistry Lab Percent Composition Chemistry Labs Teaching Chemistry Chemistry

This Is The First Time In About 7 Years That I Have Taught A Chemistry Class My Usual Teaching Assig Chemistry Labs Chemistry Education Chemistry Experiments

Ncert Solution For Class 9th Chapter 3 Atoms And Molecules Molecules Polyatomic Ion Atom

Copper Sulfate Formula Properties Uses And Structural Formula

Temperature Dependence Of The Solubility Of Copper Sulfate In Water Download Scientific Diagram

Copper Sulfate Hydrate Lab Procedure

Starch Iodine Clock Reaction Lab Kinetics Le Chatelier S Principle Le Chatelier S Principle High School Chemistry Chemistry Labs

Determining Empirical Molecular Formulas Scaffolded Notes Molecular Structural Formula Scaffolded Notes
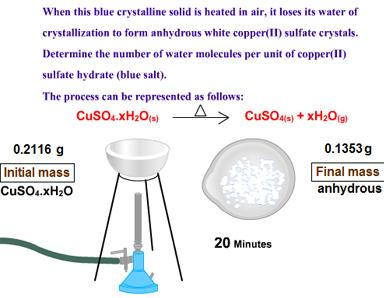
Solved A Determine The Percentage Of Water In Copper Ii Chegg Com

Precipitation And Solubility Bundle Solubility Precipitation Covalent Bonding